- Stock Photography: ELECTROLYSIS PROCESS: ANODE AND CATHODE REACTIONS by Emarandjelovic
- Price: 1$
- Size Facebook: 1702 x 630 px
- Size Twitter: 1500 x 500 px
- Size LinkedIn: 1128 x 191 px
More Facebook, Twitter and LinkedIn Cover Photos
Cover photo info
- Photo title: Electrolysis Process: Anode and Cathode Reactions
- Author: Emarandjelovic
- Cover photo description:
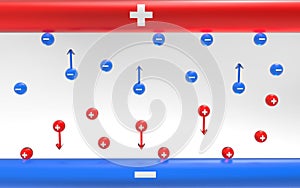
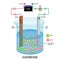
- This 3D illustration showcases the electrolysis process, focusing on the anode and cathode reactions. It demonstrates the movement of negative blue anions and positive red cations towards a metal pipe. In the center of the illustration, you can see the setup for electrolysis, with the anode and cathode placed in a solution. The anode is represented by a blue color, while the cathode is depicted in red. Negative blue anions are shown moving towards the anode, while positive red cations are depicted moving towards the cathode. The metal pipe acts as a pathway to guide the movement of these ions. This visualization helps convey the fundamental concept of electrolysis, where the application of an electric current causes chemical reactions to occur at the anode and cathode. The anode reaction involves the loss of electrons, resulting in the formation of positive ions or cations, which move towards the cathode. Conversely, at the cathode, the gain of electrons leads to the formation of negative ions or anions. The 3D rendering emphasizes the movement of ions towards the respective electrodes, highlighting the electrochemical reactions that take place during electrolysis. The use of contrasting blue and red colors helps differentiate the anions and cations, making it easier to understand the direction of ion flow.
- Image ID:133028572
- Views:14
- Downloads:1
Keywords for Facebook, Twitter and LinkedIn timeline photos
electrolysis
anode
cathode
negative
ions
positive
metal
pipe
anions
cations
electrochemical
reactions
electric
current
chemical
ion
movement
electrochemistry
redox
flow
electrolytic
process
scientific
concept
visualization
transformations
electron
transfer
electrical
charge
ionic
understanding
experimental
setup
research
demonstration
principles
investigation
education
blue
color
red
electrolyte
solution
cell
species
oxidation
reaction
reduction
electrode
migration
phenomena
transport
charge
conductivity
concentration
kinetics
potential
discovery
advancement
engineering
breakthrough
applications